by UserName | Apr 11, 2025 | Announcement
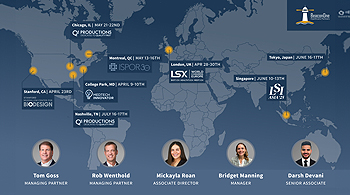
The BeaconOne Healthcare Partners team is preparing for a busy quarter of events and conferences meeting with our global network of investors and innovators. Please contact us if you would like to meet with our team at any of these...

by UserName | Feb 19, 2025 | Announcement
We are excited to welcome Hannah Peric to the Firm, as an Associate. Hannah holds dual bachelor’s degrees in Managerial Economics and Nutrition from the University of Massachusetts, Amherst. She also earned a Master of Science in Business Analytics from UMass Amherst...

by UserName | Feb 11, 2025 | Insight
NICE recognizes that the use of artificial intelligence (AI) methods, from relatively well-established machine learning approaches to newer and more complex generative AI, offers benefits such as processing and analyzing large datasets to reveal patterns and...
by UserName | Jan 30, 2025 | Insight
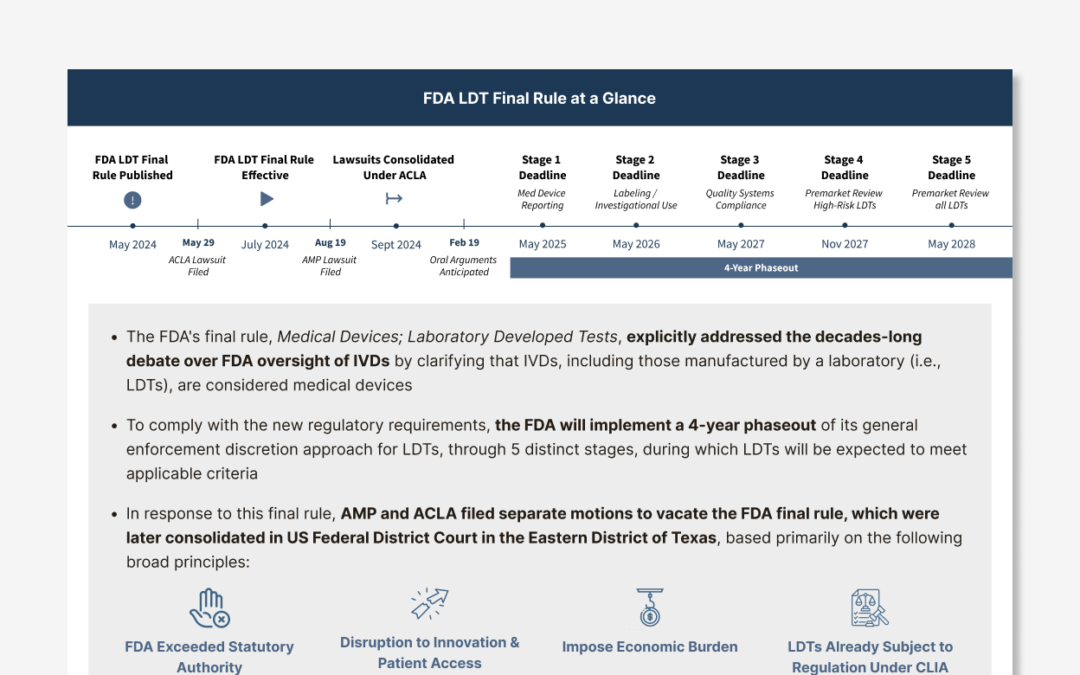
In May 2024, the decades-long debate between the FDA and CLIA program over regulation of LDTs, seemingly came to an end with the publication of an FDA final rule, making it explicit that IVDs are classified as devices under the FD&C Act, including when the...

by UserName | Oct 29, 2024 | Insight
Private payers are having to think outside the box to prioritize cost-savings for laboratory services, especially with the delayed implementation of the Protecting Access to Medicare Act (PAMA) proposed payment rate cuts to 2026, jeopardizing the integrity and...